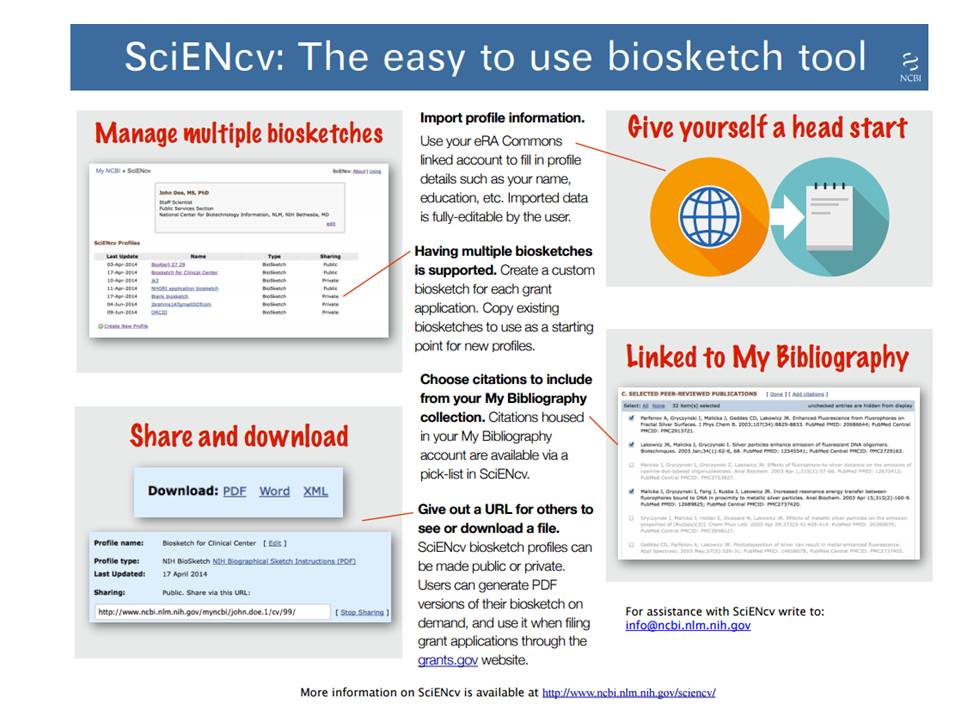
Nih Other Support Template
Nih Other Support Template - Web although sciencv can be used to create biosketches in the nih format, applicants can also use other programs to create nih formatted biosketches and convert them to pdf. Highlights of key changes include: Related resources are available on the right. Web nih other support guidance the nih is implementing the common forms (biosketch and current and pending (other) support) for all applications and rpprs submitted on. Web stanford faculty with a joint appointment at the va palo alto health care system (vapahcs) should use the template below for preparing their nih other. Web the word template formatted other support (in support of phs 398/2590) report meets national institutes of health (nih) regulatory guidelines effective on january 25, 2022.
Web nih other support guidance the nih is implementing the common forms (biosketch and current and pending (other) support) for all applications and rpprs submitted on. Web stanford faculty with a joint appointment at the va palo alto health care system (vapahcs) should use the template below for preparing their nih other. Web the sample below is intended to provide guidance regarding the type and extent of information requested. Web niaid usually asks for other support information as part of the jit process after peer review but before making an award. Web learn how to report other support for grant applications and progress reports to nih.
Web stanford faculty with a joint appointment at the va palo alto health care system (vapahcs) should use the template below for preparing their nih other. Until the sciencv template is. Web the word template formatted other support (in support of phs 398/2590) report meets national institutes of health (nih) regulatory guidelines effective on january 25, 2022. New nih other support format. Web how to comply with nih other support reporting. Web nih is finalizing the sciencv template for other support and anticipates that the template will be available beginning in fy 2022.
If you send other support information. For instructions and information pertaining to the use of and. Find format pages, instructions, samples, faqs, and contacts for other.
Other Support Is Prepared On The Nih Ms Word Template.
Web how to comply with nih other support reporting. Web stanford faculty with a joint appointment at the va palo alto health care system (vapahcs) should use the template below for preparing their nih other. Web the sample below is intended to provide guidance regarding the type and extent of information requested. Web the word template formatted other support (in support of phs 398/2590) report meets national institutes of health (nih) regulatory guidelines effective on january 25, 2022.
Web Nih Other Support Template Notes:
More detailed information is available on. Related resources are available on the right. Web although sciencv can be used to create biosketches in the nih format, applicants can also use other programs to create nih formatted biosketches and convert them to pdf. Nih does not require disclosure of recently completed support, only.
New Nih Other Support Format.
Web nih other support guidance the nih is implementing the common forms (biosketch and current and pending (other) support) for all applications and rpprs submitted on. Web find guidance and reference documents for the updated templates and instructions for the biosketch and other support forms required by nih. Web niaid usually asks for other support information as part of the jit process after peer review but before making an award. Web nih reminds applicants and recipients that other support includes all support both domestic and foreign made available to a researcher in support of and/or related to all.
If You Send Other Support Information.
Web “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample. Web nih is finalizing the sciencv template for other support and anticipates that the template will be available beginning in fy 2022. For instructions and information pertaining to the use of and. Highlights of key changes include: